

Tracers clear through lymphatic vessels at the caudal end of the spine to reach sacral and iliac LNs Finally, obvious tracer-filled vessel-like structures that were most apparent in awake mice were seen emerging from the intervertebral regions, which led us to examine the potential CSF outflow pathways from the spine in detail.
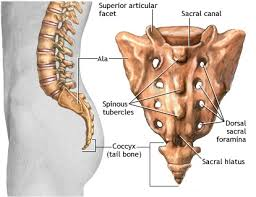
Signals continued to increase in this region over time in all three groups of mice however, signals in isoflurane-anesthetized mice were significantly higher than both the awake and ket/med-anesthetized groups at 90 min, indicating increased accumulation of tracer in this region under these conditions. 2 E), no significant differences were found at early time points between the three groups. On examination of the mean fluorescent signals at the sacral region over time ( Fig. At later time points, the number of intervertebral regions containing tracer increased, with more enhanced regions found in the awake mice compared with anesthetized groups ( Fig. First, the initial pattern of tracer signal exhibited focal accumulations in the regions between vertebrae. 2 C), several interesting observations were made. Using this approach to examine the sacral region of the spine ( Fig. Tracer accumulates at intervertebral regions in the sacral region of the spine Together, these data indicate that a directional flow of CSF occurs within the CC from the ventricular system to the caudal end of the spine. This is likely due to its low molecular weight, as EB easily diffuses into the spinal parenchyma through the ependymal lining of the CC, similar to our previous observations at the brain ventricles ( Ma et al., 2017). At the lumbar region, EB could still be detected in the CC, albeit with much weaker fluorescence ( Fig. EB was also obvious in the SAS at this level of the spine. 30 min after infusion, EB was clearly apparent in the CC of the thoracic spinal cord ( Fig. Since P40D680 is not amenable to fixation, we used Evans blue (EB) dye, which can also be assessed using its fluorescence on spinal cord sections. We next aimed to provide histological evidence of tracer within the CC of the spinal cord after i.c.v. No significant differences were apparent in the thoracic region, indicating that tracers have access to the SAS of the upper spine through either injection route (i.c.v. Signals at the sacral region were significantly higher in the i.c.v.
To test this, we compared the P40D680 tracer signals at the sacral region of spine between mice in which tracer was infused i.c.v. 1 D), implying tracer transport down the CC.Īs the entrance of the CC exists at the floor of the fourth ventricle, infusions of tracer into the lateral ventricle but not the cisterna magna should lead to tracer transport to the caudal spine ( Bradbury and Lathem, 1965). Surprisingly, the sacral region, despite its distance from the lateral ventricle infusion site, exhibited an enhancement of signal starting at an average of 17.0 ± 2.3 min after infusion that was significantly faster than the initial enhancement of 23.7 ± 5.5 min at the thoracic region ( Fig. Starting at 5 min after infusion, one group of mice was imaged at the thoracic region, while another group was imaged at the sacral region ( Fig. To evaluate this question, we employed a noninvasive dynamic imaging approach. Due to the curvature of the spine, which made these particular regions more accessible to near-infrared imaging, it was not immediately clear if the tracer had spread to the sacral region through the spinal SAS or directly through the CC from the ventricles. Two areas of the spine that we defined as thoracic and sacral regions had apparent high tracer signals ( Fig. We first aimed to evaluate the distribution of 40-kD pegylated near-infrared IRDye680 (P40D680) tracer ( Proulx et al., 2017) in the spine 60 min after intracerebroventricular (i.c.v.) infusion.


 0 kommentar(er)
0 kommentar(er)
